Pfizer, Abbott shares dip after India’s regulator bans cough syrups with codeine base

Pfizer stocks in India closed on Monday down by 9 percent in Mumbai before an Indian court granted Pfizer an interim injunction on a ban on its cough syrup Corex. The Indian Health Ministry prohibited combining codeine syrup and chlorpheniramine maleate over the weekend.
The two substances are found in Pfizer’s Corex. Prior to the court interim injunction, Pfizer stopped selling the cough syrup and anticipated a hit on its profit because the cough syrup generated sales of $26 million (AUD$34.7 million) from the 2nd to 4th quarters of 2015, reports Reuters.
But Pfizer filed a writ petition in the New Delhi High Court which granted the pharmaceutical giant some relief on Monday through a stay, pending the next court hearing on March 21. The court granted Pfizer an interim injunction because the ministry failed to give Pfizer a “show cause notice” before it banned Corex.
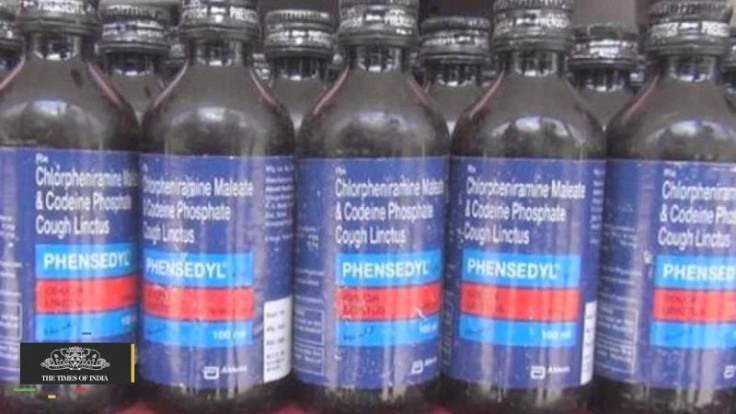
Abbot Laboratories’ Phensedyl cough syrup is also affected by the ministry’s ban. The company’s Indian subsidiary also filed a writ petition with the New Delhi High Court which would hear it on Tuesday. Abbott’s losses would have been bigger because Phensedyl has a one-third share of the Indian cough syrup market and contributes to 3 percent of Abbott’s $1 billion (AUD$1.33 billion) Indian revenue. Abbott shareprices dipped 3 percent on news of the ban.
Codeine is a narcotic, which led India to ban the substances use in cough syrups. Before the ban, the ministry pressured pharmaceutical firms to improve their policing of the supply chain to address addiction and smuggling issues. In 2015, a drug controller for Telangana, an Indian southern state, discovered illegal diversion of Phensedyl worth about $8.5 million (AUD$11.3 million).
India banned 344 fixed-dose combination drugs which a panel of experts found to lack therapeutic justification. The US Food and Drug Administration classifies codeine an opioid pain reliever used to treat mild to moderately severe pain, and in combination with other medications, to reduce coughing. It is available as a single-ingredient product or combined with acetaminophen or aspirin.





















